Life is full of grand complications, such as aerodynamic wings, multi-part organs like eyes, and intricate chemical pathways. When faced with such complexity, both opponents and proponents of evolution, Darwin included, have asked the question: how could it evolve?
Science does not sweep such difficult questions under the rug, but takes them up as interesting areas for research. The difficulty is as follows.
Since many of these complex traits seem to be adaptive, they are likely to have evolved in small steps through natural selection. That is, intermediate forms of the adaptation must have evolved before evolution arrived at a fully-fledged wing, chemical pathway, or eye. But what good is half a wing or only a few of the elements of an eyeball? The intermediate forms of these adaptations may not seem adaptive — so how could they be produced by natural selection?
There are several ways such complex novelties may evolve:
- Advantageous intermediates: It’s possible that those intermediate stages actually were advantageous, even if not in an obvious way. What good is “half an eye?” A simple eye with just a few of the components of a complex eye could still sense light and dark, like eyespots on simple flatworms do. This ability might have been advantageous for an organism with no vision at all and could have evolved through natural selection.
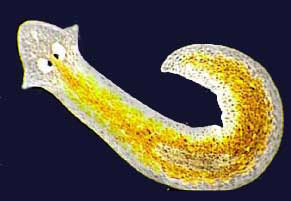
A Planaria flatworm with its light-sensitive eyespots. Photo courtesy of BioMEDIA ASSOCIATES. - Co-opting: The intermediate stages of a complex feature might have served a different purpose than the fully-fledged adaptation serves. What good is “half a wing?” Even if it’s not good for flying, it might be good for something else. The evolution of the very first feathers might have had nothing to do with flight and everything to do with insulation or display. Natural selection is an excellent thief, taking features that evolved in one context and using them for new functions.
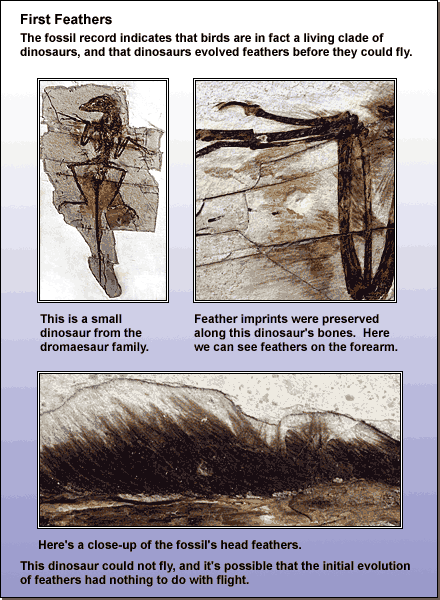
Read more about how new, complex traits evolve.
Learn more about transitional forms in context: What has the head of a crocodile and the gills of a fish?, a news brief with discussion questions.
Learn more about transitional features in Understanding macroevolution through evograms, a module exploring five examples of major evolutionary transitions in the fossil record.
Learn more about the evolution of a complex trait in context: Why the eye?, a case study.
Teach your students about the evolution of a complex trait: The evolution of flight in birds, a web activity for grades 9-12.
