In recent years, the popular media has served up a message that might make your skin crawl: lice and bedbug infestations are on the rise and are getting harder to treat as these parasites evolve resistance to the pesticides we’ve used against them in the past. But did you ever stop to wonder how they’ve invaded our beds and bodies — not how a particular outbreak started, but how ultimately we wound up with these human bloodsuckers in the first place? Last month, new research highlighted the evolutionary beginnings — and future trajectory — of bedbugs. Taking a step back reveals that the origin of new parasites is just one example of well understood evolutionary processes.
In recent years, the popular media has served up a message that might make your skin crawl: lice and bedbug infestations are on the rise and are getting harder to treat as these parasites evolve resistance to the pesticides we’ve used against them in the past. But did you ever stop to wonder how they’ve invaded our beds and bodies — not how a particular outbreak started, but how ultimately we wound up with these human bloodsuckers in the first place? Last month, new research highlighted the evolutionary beginnings — and future trajectory — of bedbugs. Taking a step back reveals that the origin of new parasites is just one example of well understood evolutionary processes.
Where's the evolution?
Parasites and pathogens don’t arise de novo from the primordial slime. They evolve from other organisms (usually from other parasites and pathogens). To understand how this happens, it helps to remember that to these bugs, the human species is a habitable ecosystem. Just as an uninhabited island can be invaded by a new species from a neighboring island, a living body can be colonized by a new bug from a nearby victim. If conditions are right and if the invader can survive and reproduce in the new host, the invader may evolve adaptations to the new host ecosystem resulting in a specialized relationship with that host over many generations. Eventually, the parasite lineage that invaded the new host may evolve so many differences from the source population, that it constitutes a separate species. In bedbugs, we are seeing this process in action.
Genetic and anatomical evidence suggest that bedbugs (Cimex lectularius) came to humans from an unexpected animal. When our early human ancestors evolved in Africa, some of them lived in caves, along with another notable cave-dweller: bats. Those bats colonies were parasitized by bloodsucking bugs. Some of the insects began to feed on their human neighbors as well. Around 250,000 years ago, the bat and human bedbug lineages began to diverge from one another. When humans left the caves and began living in other shelters, they took their parasites with them.
New research shows that the bat and human bedbug lineages — though both currently classified as the same species and capable of interbreeding — are in the process of becoming distinct species. The two lineages are broadly similar at a genetic level, but notable differences are accumulating. Of course, as we would expect since human bedbugs are frequently exposed to insecticides and bat bedbugs are not, gene versions that code for pesticide resistance are common in human bedbugs (where such gene versions would have been favored) and absent in bat bedbugs. But researchers also found a variety of differences throughout the genome. The two lineages have physical differences as well. For example, human bedbugs tend to have longer legs, and bat bedbugs have shorter, sturdier ones. Researchers have hypothesized that long legs may be an adaptation for escaping from humans or for moving between human dwellings.
It’s not surprising that that these two lineages have evolved so many distinguishing features. There is little evidence of gene flow (i.e., interbreeding) between the two lineages, and their hosts have quite different bodies and lifestyles. Humans are nearly hairless and sleep in beds at night. Bats are hairy and sleep in roosts during the day. We would expect that the traits favored by natural selection for feeding on the two hosts would be different.
Bedbugs have clearly invaded the human “island” and are here to stay, while other species have tried to make the leap and failed. Diseases like Ebola and rabies are able to invade the human “ecosystem” from their original hosts but don’t evolve enough adaptations to their new environment to set up shop permanently. For example, Ebola normally lives in bats. Every so often, the Ebola virus invades a human host (either from bats or from another wild animal infected by a bat). The virus circulates among humans for a little while, causing an outbreak, but then then eventually dies out. Such pathogens are called zoonotic infections.
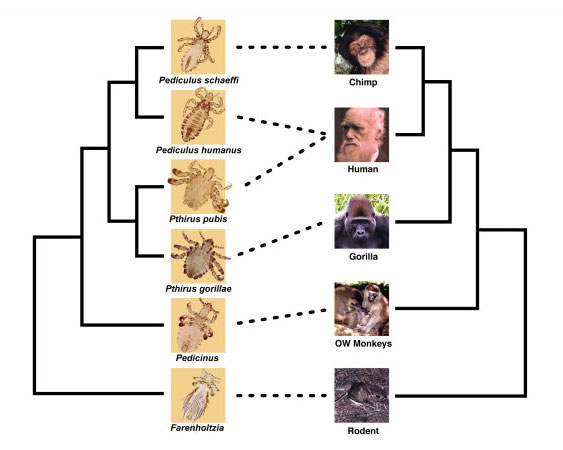
In other cases, the association between a parasite or pathogen and its host is so tight that their evolutionary paths become intertwined. Coming back to the human island analogy, imagine a large continent on which a widely distributed species lives. What would happen to that species if plate tectonics caused part of the continent to break off and move away from the rest of the landmass, forming an island? Unless the species were able to disperse over water, eventually (over many millennia) the two halves of the population would diverge and become separate species. This process is known as vicariance, and it also occurs in parasites and pathogens whose host lineage speciates, where it is known as cospeciation. For example, six million years ago, the common ancestor of humans and chimpanzees was probably sitting around in Africa scratching at the lice that lived on its hairy body. When that single ape lineage split into two, each took a group of lice with them. The early chimp ancestors had one set of lice, and the early human ancestors had another. Once those apes stopped breeding with one another, their lice had to as well, since there were no longer opportunities for the two groups of lice to mate with one another. Over many generations, as their hosts diverged, so did the lice, eventually forming the two species we have today: Pediculus humanus lives on the human head, and P. schaeffi lives on chimpanzees.
When vicariance between parasites and their hosts occurs, we can tell because their family trees line up, speciation event for speciation event. The phylogeny below shows the relationships among primates (as well as one rodent) and their lice. You can see that, for the most part, their phylogenies mirror one another: when old world monkeys split from other primates, their lice split from other lice, etc. This indicates vicariance. However, you’ll also see one place where the phylogenies don’t align. Because we have two distinct parts of the body with hair, humans have two lice species, one that lives on the head and one that lives in the pubic hair (Pthirus pubis). Our pubic lice came to us, not through vicariance, but through dispersal (as bedbugs did). Some lice from a gorilla ancestor found themselves on a human ancestor and managed to stick it out and make a living on a new host.
The evolutionary history of human parasites and pathogens is variable, but is full of familiar patterns. Some have been with us since we first became human (e.g., our head lice), some are new arrivals (e.g., our pubic lice), and some are newer still and have just begun to diverge from their closest relatives (e.g., our bedbugs). The evolutionary processes that account for these divergences and adaptations operate at many levels in the natural world — whether one is contemplating the grand sweep of evolutionary history as continents break apart, battling an Ebola outbreak in West Africa, or just nervously scratching at a red welt and eyeing one’s mattress with suspicion.
News update, July 2019
When this story was published in 2015, available evidence suggested that not only did one of the most common human bed bugs (Cimex lectularius) come to us from bats, but that the entire bed bug lineage — all 100+ species of the clade Cimicidae — got its start feeding on bat blood. Now, new research shows that those ancestral bed bugs must have been biting some other critter entirely. Researchers reconstructed the phylogeny of all the different living bed bug species based on their DNA and used a 100-million year old bed bug fossil as a calibration point to estimate when the different lineage-splitting events must have occurred. They found that the bed bug clade got its start 115 million years ago — about 30 million years before bats even existed! We don’t know which Cretaceous creature was the original unlucky recipient of bed bug bites; non-avian dinosaurs, birds, early mammals, and others were all around then. However, once bats came into the picture, bed bugs seem to have switched hosts to these warm-blooded cave-dwellers, as well as to a variety of modern birds — and finally onto humans, once we joined bats in their caves.
Primary literature:
- Booth, W., Balvin, O., Vargo, E. L., Vilimova, J., and Schal, C. (2015). Host selection drives genetic divergence in the bed bug, Cimex lectularius. Molecular Ecology. 24: 980-992. Read it »
- Reed, D. L., Light, J. E., Allen, J. M., and Kirchman, J. J. (2007). Pair of lice lost or parasites regained: the evolutionary history of anthropoid primate lice. BMC Biology. 5:7. Read it »
News articles:
- A summary of the new research on bedbugs from the New York Times
- A press release about the evolution of human lice from the University of Florida
Understanding Evolution resources:
-
- Why might we expect bedbugs living on bats and bedbugs living on humans to evolve into separate species?
- Review the process of natural selection. Use the four steps described on that page to explain how a bedbug lineage might evolve resistance to a pesticide that humans use to fight infestations.
- In your own words, explain what is meant by the term vicariance.
- Review this news brief about HIV. Is the infection of humans by HIV a case of vicariance or of switching from another host? Explain your answer.
- Advanced: In the case of vicariance, we would expect the phylogenies of parasite and host to mirror one another. How could we identify a case of host switching using the phylogenies of parasites and their hosts?
- Teach about reproductive isolation: In this classroom activity for grades 6-12, students learn about variation, reproductive isolation, natural selection, and adaptation.
- Teach about the evolution of new parasites and pathogens: This article for teachers of grades 9-12 addresses the evolution of new human diseases and comes with links to additional examples, supplementary information, and classroom tips.
- Teach about how human hosts were invaded by HIV: This multi-part case study for grades 13-16 explores changes in Simian Immunodeficiency Virus (SIV) in different chimpanzee populations and how researchers use this information to test hypotheses about the origins of HIV.
- Balvin, O., Munclinger, P., Kratochvil, L., and Vilimova, J. (2012). Mitochondrial DNA and morphology show independent evolutionary histories of bedbug Cimex lectularius (Heteroptera: Cimicidae) on bats and humans. Parasitology Research. 111: 457-469.
- Booth, W., Balvin, O., Vargo, E. L., Vilimova, J., and Schal, C. (2015). Host selection drives genetic divergence in the bedbug, Cimex lectularius. Molecular Ecology. 24: 980-992.
- Reed, D. L., Light, J. E., Allen, J. M., and Kirchman, J. J. (2007). Pair of lice lost or parasites regained: the evolutionary history of anthropoid primate lice. BMC Biology. 5:7
- Roth, S., Balvín, O., Siva-Jothy, M. T., Di lorio, O., Benda, P., Calva, O., ... Reinhardt, K. (2019). Bedbugs evolved before their bat hosts and did not co-speciate with ancient humans. Current Biology. 29: 1-7.