Geologists use radiometric dating to estimate how long ago rocks formed, and to infer the ages of fossils contained within those rocks.
Radioactive elements decay
The universe is full of naturally occurring radioactive elements. Radioactive atoms are inherently unstable; over time, radioactive “parent atoms” decay into stable “daughter atoms.”
When molten rock cools, forming what are called igneous rocks, radioactive atoms are trapped inside. Afterwards, they decay at a predictable rate. By measuring the quantity of unstable atoms left in a rock and comparing it to the quantity of stable daughter atoms in the rock, scientists can estimate the amount of time that has passed since that rock formed.
 Bracketing the fossils
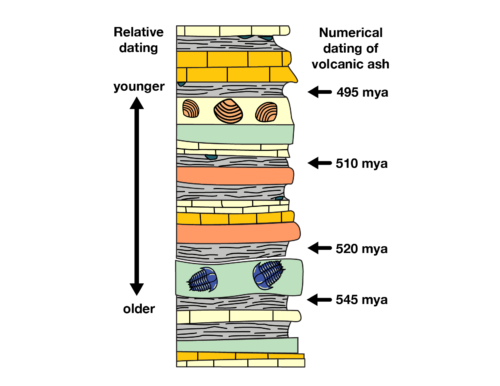
Bracketing the fossils
Fossils are generally found in sedimentary rock — not igneous rock. Sedimentary rocks can be dated using radioactive carbon, but because carbon decays relatively quickly, this only works for rocks younger than about 50 thousand years.
So in order to date most older fossils, scientists look for layers of igneous rock or volcanic ash above and below the fossil. Scientists date igneous rock using elements that are slow to decay, such as uranium and potassium. By dating these surrounding layers, they can figure out the youngest and oldest that the fossil might be; this is known as “bracketing” the age of the sedimentary layer in which the fossils occur.
Teach your students about absolute dating: Determining age of rocks and fossils, a classroom activity for grades 9-12.
Find additional lessons, activities, videos, and articles that focus on relative and absolute dating.
