This month, the World Health Organization announced that tuberculosis cases are on the decline for the first time in at least 20 years. We seem finally to be winning what has been a very long battle. Tuberculosis bacteria have been attacking us since modern humans began to migrate out of Africa around 40,000 years ago. If you enjoy classic literature, you’ll be familiar with the cough, fever, and weight loss of consumption (the old-fashioned term for tuberculosis), which used to be a near certain death sentence. That changed when the aminoglycoside antibiotic streptomycin was discovered in 1943. In combination with other drugs, streptomycin could cure tuberculosis. Though the medical community has been reconsidering how frequently it and other antibiotics of the same class should be deployed, streptomycin remains an important weapon in the fight against tuberculosis. Understanding how this drug works takes us on a fascinating evolutionary journey that begins some 1.8 billion years ago …
Where's the evolution?
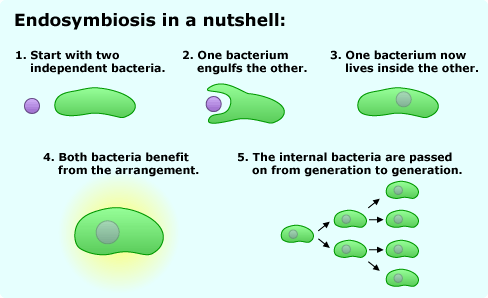
Around 1.8 billion years ago, a major divergence occurred among the single-celled organisms that then made up life on Earth. An early bacterium — one which could use oxygen to release energy from sugars — was engulfed by another bacterium. Through the process of endosymbiosis, this lineage eventually evolved into the eukaryotes (the diverse group which includes animals and plants) and the engulfed bacterium evolved into a cellular organelle (the mitochondrion, the powerhouse of the cell), while the relatives of the engulfed bacterium remained single-celled and evolved into modern bacteria.
As these two lineages — the eukaryotes and the bacteria — proceeded along their independent evolutionary paths, each acquired mutations that affected their cellular machinery. To understand the action of aminoglycoside antibiotics, the relevant cell part in each lineage is the ribosome, whose job is building proteins based on instructions from the DNA. Though all ribosomes share many basic characteristics, the eukaryotic ribosome is larger than bacterial ribosomes and is shaped somewhat differently. These differences, which began evolving so long ago, are what allow aminoglycosides to be effective antibiotics instead of dangerous toxins.
Aminoglycosides attach to the part of the bacterial ribosome that decodes messages from the DNA. By blocking protein synthesis, aminoglycosides prevent the bacterium from reproducing and carrying out its basic functions. Luckily for us, our own eukaryotic ribosomes accumulated just enough differences from bacterial ribosomes that aminoglycosides cannot attach to them and block the functioning of our own cells.
That means that aminoglycosides attack bacteria in our bodies but not the ribosomes of our own cells — with one exception. As described earlier, the mitochondria in our cells are descended from free-living bacteria, and it so happens that our mitochondria have their own ribosomes, which, as you would expect, are more similar to bacterial ribosomes than they are to the ribosomes in the rest of our cells. If aminoglycosides reach the mitochondria in our cells, they can prevent the mitochondria from functioning normally because our mitochondria have such a close evolutionary relationship with bacteria.
One consequence of aminoglycosides’ effects on mitochondrial ribosomes seems to be an occasional case of hearing loss. Apparently, in some people (especially those carrying certain mutations in the gene that codes for their mitochondrial ribosomes), the antibiotic’s effect on mitochondria in the inner ear causes the death of hair cells that play an important role in detecting sound. While it is not clear, exactly how this occurs, it does seem to be a consequence of our mitochondria’s evolutionary relationship with bacteria. Fortunately, most people seem to suffer few negative outcomes as a result of taking aminoglycosides.
Nevertheless, because of this risk (as well as another potential side effect of the drugs, kidney damage), the medical community has been reconsidering how frequently aminoclycosides such as streptomycin should be deployed. Currently, these drugs still play an important role in fighting diseases like tuberculosis and plague (yep, it’s still around!) and are an important part of our antibiotic arsenal when fighting pathogens that have evolved resistance to multiple drugs. Whatever the final verdict on their use ends up being, we have evolution to thank for both the utility of these drugs and their undesirable side effects.
Primary literature:
- Lynch, S. R., and Puglisi, J. D. (2001). Structural origins of aminoglycoside specificity for prokaryotic ribosomes. Journal of Molecular Biology. 306: 1037-1058. Read it »
- Recht, M. I., Douthwaite, S., and Puglisi, J. D. (1999). Basis for prokaryotic specificity of action of aminoglycoside antibiotics. The EMBO Journal. 18: 3133-3138. Read it »
News articles:
- A popular article on the decline in tuberculosis from from The Washington Post
- The original report on the decline in tuberculosis from the World Health Organization
- An older article on deafness caused by aminoglycosides from Medical News Today
Understanding Evolution resources:
- The article above suggests that aminoglycosides could be toxic to humans if it weren’t for our divergent evolutionary history from bacteria. Explain why.
- The article above explains how human mitochondrial ribosomes are similar to bacterial ribosomes. Do some research on mitochondria, and explain three other pieces of evidence that support the idea of a close relationship between mitochondria and free-living bacteria.
- Advanced: Some strains of tuberculosis have evolved resistance to streptomycin. Based on your understanding of how this antibiotic works, what sort of bacterial gene is likely to carry the mutation that is the basis of this resistance? Explain your reasoning.
- Advanced: Do some research on streptomycin-resistant tuberculosis strains. What is the genetic basis of this resistance? Does it match the hypothesis you formulated in the previous item?
- Teach about endosymbiosis: In this article for grades 9-16, students find out how endosymbiosis factored into the evolution of their own cells and learn about a modern example of this process.
- Teach about antibiotics and tuberculosis: In this lesson for grades 9-16, students learn why evolution is at the heart of a world health threat by investigating the increasing problem of antibiotic resistance in such menacing diseases as tuberculosis.
- Durante-Mangoni, E., Grammatikos, A., Utili, R., and Falagas, M. E. (2009). Do we still need the aminoglycosides? International Journal of Antimicrobial Agents. 33: 201-205.
- Gillespie, S. H. (2002). Evolution of drug resistance in Mycobacterium tuberculosis: clinical and molecular perspective. Antimicrobial Agents and Chemotherapy. 46: 267-274.
- Lynch, S. R., and Puglisi, J. D. (2001). Structural origins of aminoglycoside specificity for prokaryotic ribosomes. Journal of Molecular Biology. 306: 1037-1058.
- Recht, M. I., Douthwaite, S., and Puglisi, J. D. (1999). Basis for prokaryotic specificity of action of aminoglycoside antibiotics. The EMBO Journal. 18: 3133-3138.
- Selimoglu, E. (2007). Aminoglycoside-induced ototoxicity. Current Pharmaceutical Design. 13: 119-126.
- Wirth, T., Hildebrand, F., Allix-Béguec, C., Wölbeling, F., Kubica, T., Kremer, K., . . . Niemann, S. (2008). Origin, spread and demography of the Mycobacterium tuberculosis complex. PLoS Pathogens. 4: e1000160.