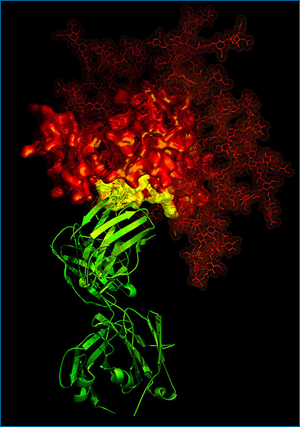
Since it made the evolutionary leap to humans from chimpanzees, HIV/AIDS has infected around 1% of the global population and in 2005 alone, killed almost three million people. Much of HIV’s continued spread can be traced to its evasion of both the human immune system and our vaccines. Now that could be changing. Last month, a team of researchers led by Peter Kwong of the National Institutes of Health revealed the details of a molecular dance between the virus and its human host cells that could pave the way for a long-awaited vaccine. The secret is in this picture. Here, an antibody (shown in green) latches onto a HIV molecule (shown in red) at its site of vulnerability (shown in yellow), thus interfering with the virus’s ability to infect new cells. What makes this yellow site HIV’s weak point? The answer is an evolutionary one…
Where's the evolution?
An HIV vaccine has been “just around the corner” for decades now, but has so far eluded researchers. HIV’s ability to slip the grasp of both our immune systems and our engineered vaccines can be blamed on the virus’s sophisticated evasive maneuvers (e.g., carbohydrate “cloaking” and “shape-shifting” identification molecules) and on its rapid evolution. To understand why HIV’s evolution is such a barrier to vaccine development, you first need to understand a bit about how the immune system works…
When your immune system comes into contact with an invader, it produces antibodies tailor-made to recognize molecules on the surface of that invader. The antibodies latch onto the invader to help neutralize it or to mark it for destruction by other parts of the immune system. Preventative vaccines work by stimulating your body to produce the antibodies specific to a particular invader before you encounter the disease, priming your body with defensive weapons before it is ever attacked. But in order to fend off the invader, the body must be prepared with exactly the right antibodies, because of the lock-and-key relationship between the antibody and the surface molecule it recognizes. Different antibodies fit different surface molecules: polio antibodies fit the polio virus, smallpox antibodies fit the smallpox virus — and this is where the evolutionary rub comes in…
Viruses like HIV have some of the highest mutation rates known — and this high mutation rate means that HIV evolves quickly. In fact, HIV evolves so quickly that its surface molecules are a constantly moving target — and our immune systems (and vaccine developers) simply can’t keep up. As it replicates within a single infected individual, HIV accumulates mutations that change the shape of its surface proteins, evolving right out from under the antibodies produced by the victim’s immune system. Similarly, as the virus is passed from person to person, it diversifies further, evolving into many different strains with differently shaped surface proteins. And all of those different strains spreading in the human population present a challenge to medical researchers: how to develop a vaccine that works for a myriad of viral versions.
Now, researchers like Peter Kwong and his team are narrowing in on what might be the key to a vaccine: a region of HIV’s surface protein that is so important to the virus’s survival that it is evolutionarily conserved. The protein is called gp120 (shown in the first image here in red and yellow), and its job is to help HIV invade human cells. The first step of HIV’s invasion involves a molecular interaction — a “handshake” — between a small part of gp120 (shown in yellow) and the victim cell. That handshake kicks off a series of interactions, which ultimately allow the virus to invade the host and hijack its DNA in order to make more HIV viruses.
The shape of the “handshake” region of gp120 is key: any HIV virus that happens to carry a mutation changing the shape of the vital region will be unable to invade a host cell and replicate itself. Because they hinder the virus’s ability to reproduce, natural selection immediately eliminates such mutations from the viral population. So while other parts of HIV’s surface are free to evolve new shapes, this key region of HIV-1 remains the same and is evolutionarily conserved. Furthermore, Kwong’s team has shown that this region seems to be exposed in an unguarded position on the molecular surface — unlike other key regions, which are hidden and protected by the shape-shifting gp120 protein. In fact, this “handshake” site seems to be vulnerable to a powerful antibody, b12 (shown in the first image here in green). B12 is one of a handful of known antibodies capable of decommissioning many different HIV variants and was first isolated from an HIV patient whose immune system was unusually successful at slowing the progress of the virus.
So is this region of gp120 HIV’s Achilles heel? Can the success of the antibody b12 against this region be leveraged into a successful vaccine? Only research and time will tell, but biologists are hopeful. After all, if HIV’s deadly evasion of our immune systems can be traced to the virus’s rapid evolution, perhaps the key to controlling the epidemic is tucked into the part of the virus that does not evolve.
News update, August 2011
Since narrowing in on the conserved region of HIV’s gp120 protein in 2007, researchers have been pursuing a vaccine that exploits this vulnerability. Scientists knew that antibody b12 attacks this site and can neutralize about 40% of HIV-1 strains, but could more effective antibodies be found? To answer this question, a group of researchers began a search for other antibodies that bind to the key region of gp120, in the process filtering through 25 million white blood cells from HIV positive people whose bodies were particularly good at keeping their infections at bay. In 2010, the researchers announced their most impressive result: an antibody that blocks 90% of common HIV-1 strains! The newly identified antibody, called VRC01, seems to mimic the interaction of the host’s immune system cells with HIV as they are being invaded by the virus. Of course, finding this antibody is still a long way from a producing a working vaccine. Vaccines trigger a person’s body to make defensive antibodies before the individual is ever infected with the disease. We still have to figure out how to manage this trick with VRC01 and other powerful antibodies — a daunting problem, but one that scientists all over the world are trying to solve. As new milestones in this process are reached, we’ll keep you updated — so stay tuned!
Primary literature:
- Saphire, E. O., Parren, P. W. H. I., Pantophlet, R., Zwick, M. B., Morris, G. M., Rudd, P. M., Dwek, R. A., Stanfield, R. L., Burton, D. R., and Wilson, I. A. (2001). Crystal structure of a neutralizing human IgG against HIV-1: A template for vaccine design. Science 293:1155-1159.
- Wu, X., Yang, Z., Li, Y., Hogerkorp, C., Schief, W. R., Seaman, M. S., . . . Mascola, J. R. (2010). Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 329: 856-861. Read it »
- Zhou, T., Xu, L., Dey, B., Hessell, A. J., Van Ryk, D., Xiang, S., Yang, X., Zhang, M., Zwick, M., Arthos, J., Burton, D. R., Dimitrov, D. S., Sodroski, J., Wyatt, R., Nabel, G. J., and Kwong, P. D. (2007). Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature 445:732-737. Read it »
News articles:
- A press release about the discovery from NIH News
- Detailed coverage of the research from The Independent
- A review of how HIV infects cells from Johns Hopkins, along with a flash animation
- A report on the discovery of VRC01 from The Wall Street Journal
Understanding Evolution resources:
- How does HIV’s mutation rate affect its rate of evolution?
- Medical researchers have had trouble developing an effective HIV vaccine for several reasons. Explain how and why HIV’s rapid evolution makes vaccine development difficult.
- This article describes the “handshake” region of gp120 as being “evolutionarily conserved.” Explain what that means and why some regions of the molecule are conserved while others are not.
- Imagine that you are studying a protein in a population of fruit flies. The job of the protein is to bind to oxygen molecules and carry them to the cells of the fly. You notice that much of the protein varies a lot from fly to fly — but one part of the protein seems to be exactly the same in all the flies that you study. What might you conclude about that unchanging part of the protein? Based on this limited information, formulate a hypothesis about the function of the unchanging region.
- Read this article about HIV evolution. Describe the similarities between the difficulties of treating HIV with drugs and the difficulties that our immune systems have fighting HIV.
- Teach about applications of evolutionary biology in medicine: This tutorial for grades 9-12 explores just a few of the many cases in which evolutionary theory helps us understand and treat disease. Bacterial infections, HIV, and Huntington's disease are highlighted.
- Teach about rapid evolution and medical applications: In this classroom activity for grades 9-12, students learn why evolution is at the heart of a world health threat by investigating the increasing problem of antibiotic resistance in such menacing diseases as tuberculosis.
- Teach about HIV evolution: In this article for grades 9-12, students learn about the role of phylogenetic evidence in a Libyan court case. Six medical workers have been convicted of injecting children with HIV-tainted blood, but the evolutionary history of the virus paints a different picture.
- Ledford, H. (2010) Souped-up antibody fends off HIV. Nature. Retrieved August 2, 2011 from Nature News.
- National Institute of Allergy and Infectious Diseases. (2007, February 14). Scientists unveil piece of HIV protein that may be key to AIDS vaccine development. NIH News. Retrieved February 27, 2007 from http://www.nih.gov/news/pr/feb2007/niaid-14.htm.
- Saphire, E. O., Parren, P. W. H. I., Pantophlet, R., Zwick, M. B., Morris, G. M., Rudd, P. M., Dwek, R. A., Stanfield, R. L., Burton, D. R., and Wilson, I. A. (2001). Crystal structure of a neutralizing human IgG against HIV-1: A template for vaccine design. Science 293:1155-1159.
- UNAIDS. (2006). 2006 report on the global AIDS epidemic: Executive summary. Geneva: UNAIDS.
- Wu, X., Yang, Z., Li, Y., Hogerkorp, C., Schief, W. R., Seaman, M. S., . . . Mascola, J. R. (2010). Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 329: 856-861.
- Zhou, T., Xu, L., Dey, B., Hessell, A. J., Van Ryk, D., Xiang, S., Yang, X., Zhang, M., Zwick, M., Arthos, J., Burton, D. R., Dimitrov, D. S., Sodroski, J., Wyatt, R., Nabel, G. J., and Kwong, P. D. (2007). Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature 445:732-737.
