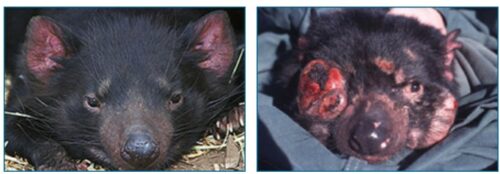
If you’ve seen images of it on the news or in the paper, you won’t soon forget it. Devil facial tumor disease (DFTD) causes bulging cancerous lumps and lesions to erupt around the face and neck — often causing enough deformation to make seeing or eating difficult. While it may be something of a relief to learn that this fatal disease affects only Tasmanian devils, marsupial carnivores of Tasmania, its impact on that population has been staggering. The disease was first observed by a wildlife photographer in 1996 and, since then, has reduced the total devil population by half — and in some areas, by as much as 90%! Tasmanian devils were recently listed as endangered and could become extinct in the wild in the next few decades. This summer, however, scientists reported that devils may be responding to DFTD by breeding earlier — before they are likely to be killed by the disease. This change could help the species survive longer, but is it an evolutionary one?
Where's the evolution?
It’s not yet clear if devils are actually evolving in response to DFTD. Scientists have observed that, before the disease, most females began breeding at two years of age and that many devil females now begin to reproduce at just one year. What’s the explanation for these observations? It could be that DFTD has selected for younger-breeding females. After all, few devils now survive to their normal breeding age. Females with genes for early-breeding would have a significant reproductive advantage over females with genes for standard breeding times, and because of this differential reproduction, the population may have evolved. Alternatively, it could be that the genetic makeup of the population has not changed, but that with reduced competition from older females and with more access to food because of diminished devil populations, younger females are now able to breed. The idea here is that devil populations always had the potential for early breeding but that this was previously suppressed by competition from other devils. This second hypothesis relies on a phenomenon known as phenotypic plasticity — alternate traits an organism might have depending, not on different gene versions, but on the organism’s current or past environment.
Further research will be needed to determine whether the shift in devil breeding times can be chalked up to evolution or to phenotypic plasticity. But even if phenotypic plasticity is the culprit, the marks of evolution on this story are deep. Perhaps most fascinating is the evolution of DFTD itself. Normally, cancer evolves within one patient. A cell happens to accumulate a series of mutations that allow it to obtain extra resources from the body and multiply more quickly than its neighboring cells. Because daughter cells inherit those same mutations for rampant proliferation, the mutant cell types become more common in the body through the process of natural selection. Over time, mutations that further increase this cell lineage’s rate of propagation or that increase its ability to survive challenges we throw at it — like chemotherapy — will be similarly favored. But of course, evolution has no foresight. If not stopped by the body or medical treatments, the evolving cell lineage may kill its “host” and, in the process, itself.
DFTD, however, has evolved an ability that is almost unique among cancers — the power to dodge the death of its host by infecting a new animal: DFTD is transmissible. It has evolved into a contagious cancer. Devil mating behavior involves biting around the head and neck, allowing cells from one individual — especially cells from the crumbly DFTD tumors — to be transferred to the wounds or face of a new individual. There, the cells begin to sap the new body’s resources and divert those nutrients toward their own proliferation, continuing their deadly advance through the devil population. This transmission results in a strange irony. The cells in DFTD tumors are more closely related to each other than they are to the other body cells in the animal of which they are a part. Though DFTD is technically a cancer, it behaves more like a parasite.
Human cancers, along with almost all cancers, are not themselves contagious because cells that somehow invade a new body are recognized as “foreign” and attacked by the immune system of the would-be host. Our bodies have a remarkable ability to distinguish self from non-self, based on genetically encoded markers on cell surfaces. Non-self markers tag a trespassing cell for destruction. This is part of the reason that transplanting organs usually requires careful genetic matching. In Tasmanian devils, however, this normal immune response does not kick in and oust foreign DFTD cells. That’s because these key cell markers (technically known as major histocompatibility molecules), which normally vary widely between individuals, are remarkably uniform among devils. Tumor cells from distantly related devils bear markers similar to a new host’s own markers — and so the tumor cells are able to fly under the radar of the host’s immune system.
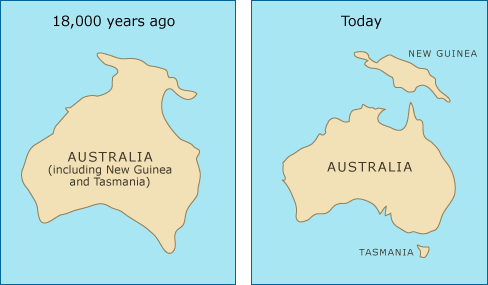
How did the devils wind up with such invariable immune system markers? What opened the door for this mutant cell lineage to evolve a life of its own, transcending the lifetime of any individual devil? The answer here depends on the other side of the evolutionary equation: the Tasmanian devil’s history. Devils used to be broadly distributed across Australia. When sea levels rose 12,000 years ago, a small number of devils on Tasmania were cut off from the mainland population, which soon went extinct. This founding population of modern Tasmanian devils did not have as many different gene versions as the larger population had had, resulting in a serious cutback in genetic variation. This is an example of the founder effect — changes in gene frequencies that usually accompany founding a new population from a small number of individuals. Though the Tasmanian population grew in numbers after it was isolated, it was stuck with a low level of genetic variation and passed this deficiency on to modern devil populations. Because there is little variation in the genes that form the basis of their immune response — and because their aggressive breeding behavior allows cell exchange — devils provided a unique opportunity for the evolution of a contagious cancer.
DFTD cells are so successful at multiplying and spreading that they may shortly bring about their own demise. The Tasmanian devil could soon be on the brink of extinction in the wild — and if all the devils die, so too will the cancer. But extinction is not the only possible end to this story. As we heard in the news this summer, the devils may be evolving in response to the disease. Natural selection favors gene variants that help the devils survive the disease and reproduce. Those favorable gene variants might underlie shifts in life history strategies (as recent research hints at), in behavior, or in how the devils’ immune systems fight the cancer. Evolution may yet stumble upon a solution for the devils. In the meantime, biologists are working to develop a vaccine and to curb the cancer by culling or isolating infected devils. But this is a race against time, and it’s not yet clear whether the biologists’ interventions or the devils’ continuing evolution will come fast enough to save them from extinction.DFTD cells are so successful at multiplying and spreading that they may shortly bring about their own demise. The Tasmanian devil could soon be on the brink of extinction in the wild — and if all the devils die, so too will the cancer. But extinction is not the only possible end to this story. As we heard in the news this summer, the devils may be evolving in response to the disease. Natural selection favors gene variants that help the devils survive the disease and reproduce. Those favorable gene variants might underlie shifts in life history strategies (as recent research hints at), in behavior, or in how the devils’ immune systems fight the cancer. Evolution may yet stumble upon a solution for the devils. In the meantime, biologists are working to develop a vaccine and to curb the cancer by culling or isolating infected devils. But this is a race against time, and it’s not yet clear whether the biologists’ interventions or the devils’ continuing evolution will come fast enough to save them from extinction.
News update, June 2013
Since 2008, scientists studying Tasmanian devils have been trying to figure out how to stop DFTD (devil facial tumor disease) before it drives the animals to extinction in the wild. While no magic bullets have been discovered, in the last five years, scientists have made significant progress in understanding how the disease arose and operates.
First off, the disease seems to have arisen in a female devil 10 to 20 years ago. Mutations occurred in a cell in her nervous system (probably a Schwann cell) that transformed it into a cancer. As described in the article above, scientists had hypothesized that DFTD was able to spread from devil to devil because of low levels of genetic variation among the animals. The idea was that devils might be so genetically similar to one another that their immune systems would not recognize the cancer cells passed from another devil as foreign and so would treat the cells as part of the devil’s own body. However, recent experiments have shown that devils’ immune systems can recognize and launch an attack against normal cells from other devils, so this doesn’t seem to be an issue with lack of diversity making it easy for cancer cells to fly under the radar. Instead, it seems that cancer cells have special characteristics that help them evade detection. Diversity at immune system genes may still influence susceptibility to the cancer, but the story clearly isn’t as simple as the original hypothesis. Devils aren’t sitting ducks. Instead, the cancer seems to have specific adaptations that allow it to outwit the immune system.
Ongoing genetic studies may help identify these adaptations. In 2012, researchers announced that they had sequenced the DNA of DFTD cells taken from two different animals and compared them to the devil genome. The research team identified about 20,000 mutations that distinguish a normal devil cell from an invasive cancer cell. Somewhere in those mutations may lie the secret to DFTD’s success — and with it, perhaps, an Achilles heel that could be used to attack the cancer — but sorting through all those mutations to figure out which are important will be a challenging task.
Another lead for heading off the cancer lies in a group of devils from Northwest Tasmania which seem to be resistant to the disease. In many locations, cancer has reduced devil populations by 90% or more, but in this area, the toll is much lower — around 20%. Scientists are currently trying to figure out why. If the difference is a genetic trait specific to these animals, we might be able to harness the same mechanism to treat or prevent the disease.
Scientists are working on many fronts to understand DFTD. But it is a race against time. If the cancer continues its rampage, devils will be extinct in the wild within in the next 35 years. Just in case, conservation programs are working to establish cancer-free preserves for the animals on other islands and in zoos and sanctuaries. If the disease cannot be stopped, once it has entirely run its course, mainland Tasmania could be repopulated with devils from these preserves. Stay tuned to find out if new research yields any new strategies for battling this contagious cancer!
Primary literature:
- Jones, M. E., Cockburn, A., Hamede, R., Hawkins, C., Hesterman, H., Lachish, S., Mann, D., McCallum, H., and Pemberton, D. (2008). Life-history change in disease-ravaged Tasmanian devil populations. Proceedings of the National Academy of Sciences 105:10023-10027. Read it »
- Jones, M. E., Paetkau, D., Geffen, E., and Moritz, C. (2004). Genetic diversity and population structure of Tasmanian devils, the largest marsupial carnivore. Molecular Ecology 13(8):2197-2209. Read it »
- McCallum, H., Tompkins, D. M., Jones, M., Lachish, S., Marvanek, S., Lazenby, B., Hocking, G., Wiersma, J., and Hawkins, C. E. (2007). Distribution and impacts of Tasmanian Devil Facial Tumor Disease. EcoHealth 4(3):318-325.
- Siddle, H.V., Kreiss, A., Eldridge, M. D. B., Noonan, E., Clark, C. J., Pyecroft, S., Woods, G. M., and Belov, K. (2007). Transmission of a fatal clonal tumor by biting occurs due to depleted MHC diversity in a threatened carnivorous marsupial. Proceedings of the National Academy of Sciences 104(41):16221-16226. Read it »
News articles:
- A brief report on recent shifts in devil breeding times from Science News
- A thorough review of the evolution of DFTD from Harper's Magazine
- A summary of the links between evolution and cancer from Howard Hughes Medical Institute
- A review of recent research on DFTD from the New York Times
Understanding Evolution resources:
- Explain the concept of phenotypic plasticity and list three examples of phenotypic plasticity not mentioned in this article.
- Scientists are not yet sure whether devils are evolving in response to DFTD. Do some research on the Understanding Evolution website, and describe at least two other examples in which a modern organism seem to be evolving in response to pathogens, changes in the environment, or human activities.
- We normally think of evolution acting on species or populations. Explain how the concept of evolution can apply to cell lineages. What are the parallels between populations and cell lineages that allow them both to evolve?
- Read about bottlenecks and founder effects. In your own words, explain what a founder effect is and how it affects a population’s level of genetic variation.
- This article explains how a cancer in Tasmanian devils evolved to be contagious. Do you think that this same phenomenon is likely to occur with a human cancer? Explain your reasoning and list at least two reasons that support your view. Be sure to consider means of transmission and genetic variation in your response.
- Teach about natural selection: In this classroom activity for grades 9-12, students learn why evolution is at the heart of a world health threat by investigating the increasing problem of antibiotic resistance in such menacing diseases as tuberculosis.
- Teach about evolution and conservation: This lesson for grades 9-12 uses paper chromatography to simulate electrophoresis of DNA. The problem posed is to identify the genetic similarities among several sub-species of wolf in order to provide information for a conservation/breeding program.
- Teach about founder effects: In this lesson for grades 9-12, students achieve an understanding of the Hardy-Weinberg Equilibrium by using decks of playing cards without recourse to algebra. Extensions provided will facilitate the understanding of genetic drift and the founder effect.
- Jones, M. E., Cockburn, A., Hamede, R., Hawkins, C., Hesterman, H., Lachish, S., Mann, D., McCallum, H., and Pemberton, D. (2008). Life-history change in disease-ravaged Tasmanian devil populations. Proceedings of the National Academy of Sciences 105:10023-10027.
- Jones, M. E., Paetkau, D., Geffen, E., and Moritz, C. (2004). Genetic diversity and population structure of Tasmanian devils, the largest marsupial carnivore. Molecular Ecology 13(8):2197-2209.
- Kreiss, A., Cheng, Y., Kimble, F., Wells, B., Donovan, S., Belov, K., and Woods, G. M. (2011). Allorecognition in the Tasmanian devil (Sarcophilus harrisii), an endangered marsupial species with limited genetic diversity. PLoS One. 6: e22402.
- Lane, A., Cheng, Y., Wright, B., Hamede, R., Levan, L., Jones, M., Ujvari, B., and Belov, K. (2012). New insights into the role of MHC diversity in Devil Facial Tumour Disease. PLoS One. 7: e36955.
- McCallum, H., Tompkins, D. M., Jones, M., Lachish, S., Marvanek, S., Lazenby, B., Hocking, G., Wiersma, J., and Hawkins, C. E. (2007). Distribution and impacts of Tasmanian Devil Facial Tumor Disease. EcoHealth 4(3):318-325.
- Murchison, E. P., Schulz-Trieglaff, O. B., Ning, Z., Alexandrov, L. B., Bauer, M. J., Fu, B. ... Stratton, M. R. (2012). Genome sequencing and analysis of the Tasmanian devil and its transmissible cancer. Cell. 148: 780-791.
- Quammen, D. (2008, April). Contagious cancer: the evolution of a killer. Harper's Magazine. 33-43.
- Siddle, H.V., Kreiss, A., Eldridge, M. D. B., Noonan, E., Clark, C. J., Pyecroft, S., Woods, G. M., and Belov, K. (2007). Transmission of a fatal clonal tumor by biting occurs due to depleted MHC diversity in a threatened carnivorous marsupial. Proceedings of the National Academy of Sciences 104(41):16221-16226.
