
One more COVID story, and then next month we promise to bring you something different. Evolution underlies much research on SARS-CoV-2 so it keeps popping up in important and fascinating ways:
Since the beginning of the pandemic, scientists have wondered why SARS-CoV-2 affects people so differently. Some wind up on life support or die, while others don’t even notice they are infected. Most factors that tip this balance turned out to be more environmental than evolutionary. We now know that smoking, obesity, and conditions like cancer and diabetes can make COVID-19 more dangerous. Other research highlights how social inequalities and racial disparities contribute to higher infection, hospitalization, and death rates in some groups (e.g., Black and Hispanic Americans). However, studies have also revealed gene variants associated with increased risk, and one set of these has a deep evolutionary history among not just humans, but our ancient relatives: Neanderthals.
Where's the evolution?
Research published over the summer showed that carrying a particular sequence on a stretch of DNA — about 50,000 bases located on human chromosome 3 — increases one’s odds of being hospitalized during a COVID-19 infection by about 60%. Now, we know that that genetic sequence came to us from Neanderthals. Of course, Neanderthals are not the ancestors of modern humans. We did not inherit this gene sequence from them like you might inherit brown eye color from your grandmother. Instead, Neanderthals are our evolutionary cousins; they, along with Denisovans, another lineage of early humans, coexisted on Earth for hundreds of thousands of years before Neanderthals and Denisovans went extinct.
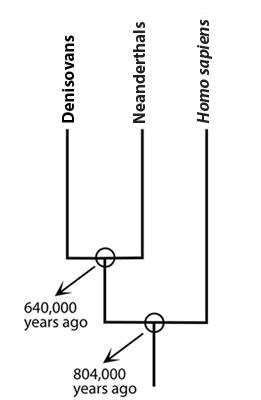 These three lineages mainly (though not always) lived in different regions: humans in Africa, Neanderthals in western Eurasia, and Denisovans further east. When they did come into contact — like when modern humans migrated out of Africa — they interbred. This interbreeding was not substantial enough that the lineages merged, but it did leave a genetic signature on descendants. Ancient Neanderthal DNA contains some human genetic sequences, ancient Denisovan DNA has some Neanderthal genetic sequences, and modern human genomes contain DNA sequences from both Neanderthals and Denisovans. The movement of genetic sequences into populations through migration and interbreeding is known as gene flow. And this is how a stretch of Neanderthal DNA wound up being passed down through human lineages over tens of thousands of years and, ultimately, landed some of us in the hospital with COVID-19.
These three lineages mainly (though not always) lived in different regions: humans in Africa, Neanderthals in western Eurasia, and Denisovans further east. When they did come into contact — like when modern humans migrated out of Africa — they interbred. This interbreeding was not substantial enough that the lineages merged, but it did leave a genetic signature on descendants. Ancient Neanderthal DNA contains some human genetic sequences, ancient Denisovan DNA has some Neanderthal genetic sequences, and modern human genomes contain DNA sequences from both Neanderthals and Denisovans. The movement of genetic sequences into populations through migration and interbreeding is known as gene flow. And this is how a stretch of Neanderthal DNA wound up being passed down through human lineages over tens of thousands of years and, ultimately, landed some of us in the hospital with COVID-19.
Different human populations have different amounts of Neanderthal ancestry. African populations have the least, as their ancestors never directly encountered Neanderthals and instead got their Neanderthal DNA from humans returning to Africa. Other human populations have more Neanderthal ancestry, ranging between 2 and 6% of the genome, with East Asians having the most. Importantly, different people carry different subsets of Neanderthal genes, and some genes are more common than others. This means that the distribution of the COVID-19 risk factor genetic sequence does not align with overall Neanderthal ancestry. The gene sequence is very rare in people of African and East Asian descent, is carried by 9% of Americans of mixed background, 16% of Europeans, and 50% of those with South Asian ancestry. The risky sequence is particularly common in Bangladesh, where 63% of the population carries at least one copy.
The distribution of the genetic sequence among populations might help explain some observations, like why, in the United Kingdom, those of Bangladeshi descent are more than twice as likely to be killed by COVID-19 than the rest of the population. And of course, it leaves other statistics glaringly unexplained. The risky sequence is much more common in Europe than in Africa, yet in the U.S., African Americans are more than five times as likely as white people of European descent to be hospitalized with COVID-19. Clearly, other powerful and concerning forces are also at work.
In general, Neanderthal genes that are commonly found in South Asian populations are also common in East Asians, and Neanderthal genes that are rare in one are also rare in the other. However, this sequence is different: it is common in South Asia, but rare in East Asia. This unusual pattern could be explained by natural selection favoring the sequence in South Asia and/or stamping it out in East Asia. There are several plausible hypotheses. Perhaps the sequence was favored in South Asia because it heightened the body’s immune response and made carriers less likely to die in ancient pandemics — but in response to SARS-CoV-2, the sequence contributes to the potentially deadly, over-the-top immune response that seems to occur in the most severe cases of COVID-19. Or perhaps the sequence has always put one at risk during a coronavirus infection and ancestral populations in East Asia were more frequently infected with coronaviruses, selecting against the sequence in that region. Additional studies of what the genetic sequence actually does should help clarify why it is more common some places and contribute to our understanding of how SARS-CoV-2 causes disease so that we can better fight it.
So what should we make of all this? Human evolutionary history impacts our health in many ways every day. For example, it can help explain why we might crave sugar and fat even when we feel full, why allergies and asthma have become more prevalent in some countries, and why diseases like sickle cell anemia are more common in people whose ancestors hail from particular regions. The new research into COVID-19 risk and how it intersects with our Neanderthal ancestry is just one more example highlighting viewing humans as a product of a complex evolutionary past, which they still bear the marks of, can illuminate and help solve medical problems.
News update, July 2022
Since we first reported on this story, scientists have continued to investigate the snippet of Neanderthal DNA that made its way into some modern humans and makes them more vulnerable to COVID-19. The latest research shows that this bit of Neanderthal DNA affects how certain proteins on the surface of our immune cells are expressed – in particular, the protein CCR5, which HIV uses to get into cells. The scientist behind the new work wondered if the Neanderthal DNA might also impact HIV infection. Indeed, he found evidence that the same stretch of DNA that makes one more likely to be hospitalized with COVID-19 also seems to provide protection against being infected by HIV. This genetic variant became common among humans 10-20,000 years ago, but it’s still not clear why. One hypothesis is that it also provides protection against smallpox, which had already jumped to humans from another host (likely a rodent), and so was favored by natural selection.
Primary literature:
- The Severe Covid-19 GWAS Group. Genomewide association study of severe Covid-19 with respiratory failure. (2020). New England Journal of Medicine. 383: 1522-1534. Read it »
- Zeberg, H., and Pääbo, S. (2020). The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature. DOI: https://doi.org/10.1038/s41586-020-2818-3 Read it »
News articles:
- A 60 second audio report on the topic from Scientific American
- An in-depth review of the research on genetic factors that influence COVID-19 severity from Science Magazine
Understanding Evolution resources:
- Review the concepts of genotype and phenotype. The article above focuses on a genotype inherited from Neanderthals.
- What is the relevant phenotype that this genotype influences?
- Is this phenotype influenced by environmental factors as well? Explain your answer.
- In your own words, explain what gene flow is.
- Using the concept of gene flow, explain how the genetic risk factor for severe COVID-19 wound up in humans.
- Do some research online and describe another example of a gene in humans with ancestry that has been traced to an early human relative.
- The article above describes how the frequency of a genetic sequence that we inherited from Neanderthals might have been affected by natural selection in the past. Do you think the frequency of this sequence is being affected by natural selection today? Why or why not? If you answered yes, how is it being affected?
- Teach about human evolution: In this lab for grades 9-16, students describe, measure, and compare cranial casts from contemporary apes, modern humans, and fossil hominids to discover some of the similarities and differences among these forms and to see the pattern leading to modern humans.
- Teach about our relatedness to Neanderthals: In this online activity for grades 9-12, students compare the number of mutations in the mitochondrial genomes of Neanderthals and humans to determine ancestry and relatedness.
- Teach about another genetic variant we inherited from Neanderthals: In this news brief for grades 9-16, students learn about a gene that influences diabetes risk and was inherited from Neanderthals.
- Centers for Disease Control and Prevention. (August 18, 2020). COVID-19 hospitalization and death by race/ethnicity. Retrieved October 30, 2020 from https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
- Chen, L., Wolf, A. B., Fu, W., Li, L., and Akay, J. M., (2020). Identifying and interpreting apparent Neanderthal ancestry in African individuals. Cell. 180: 677-687.
- Kuhlwilm, M., Gronau, I., Hubisz, M. J., de Filippo, C., Prado-Martinez, J., Kircher, M., ... Castellano, S. (2016). Ancient gene flow from early modern humans into Eastern Neanderthals. Nature. 530: 429-433.
- Li, Y., Carroll, D. S., Gardner, S. N., and Damon, I. K. (2007). On the origin of smallpox: correlating variola phylogenics with historical smallpox records. Proceedings of the National Academy of Sciences. 104: 15787-15792.
- Luo, Y. (2020). Neanderthal DNA highlights complexity of COVID risk factors. Nature. DOI: https://doi.org/10.1038/d41586-020-02957-3
- The Severe Covid-19 GWAS Group. (2020). Genomewide association study of severe Covid-19 with respiratory failure. New England Journal of Medicine. 383: 1522-1534.
- Zeberg, H. (2022). The major genetic risk factor for severe COVID-19 is associated with protection against HIV. Proceedings of the National Academy of Sciences. 119: e2116435119.
- Zeberg, H., and Pääbo, S. (2020). The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature. DOI: https://doi.org/10.1038/s41586-020-2818-3
Translated by Fran Guerola
