If you’ve scrolled past the political news lately, you may have noticed increasingly alarming stories about bird flu, a virus that mainly affects birds but can also infect humans and other animals. Outbreaks on farms have led to the preventative killing of more than a hundred million chickens, turkeys, and other farm birds, driving up the cost of eggs. The virus has also spread to dairy cows across the country. One in five samples of supermarket milk have evidence of deactivated virus, and infectious virus particles have been found in raw milk. Contaminated pet food has led to the death of cats, and zoo animals have died after contracting the virus, perhaps from wild birds that landed in their enclosures. Multiple U.S. farmworkers have been infected, likely through contact with farm animals, and the United States recently reported its first death associated with the virus. You may well be asking, is this COVID all over again? Are we on the precipice of the next pandemic? Here, we’ll use the lens of evolution to compare the virus responsible for COVID-19 to the virus behind bird flu.
Where's the evolution?
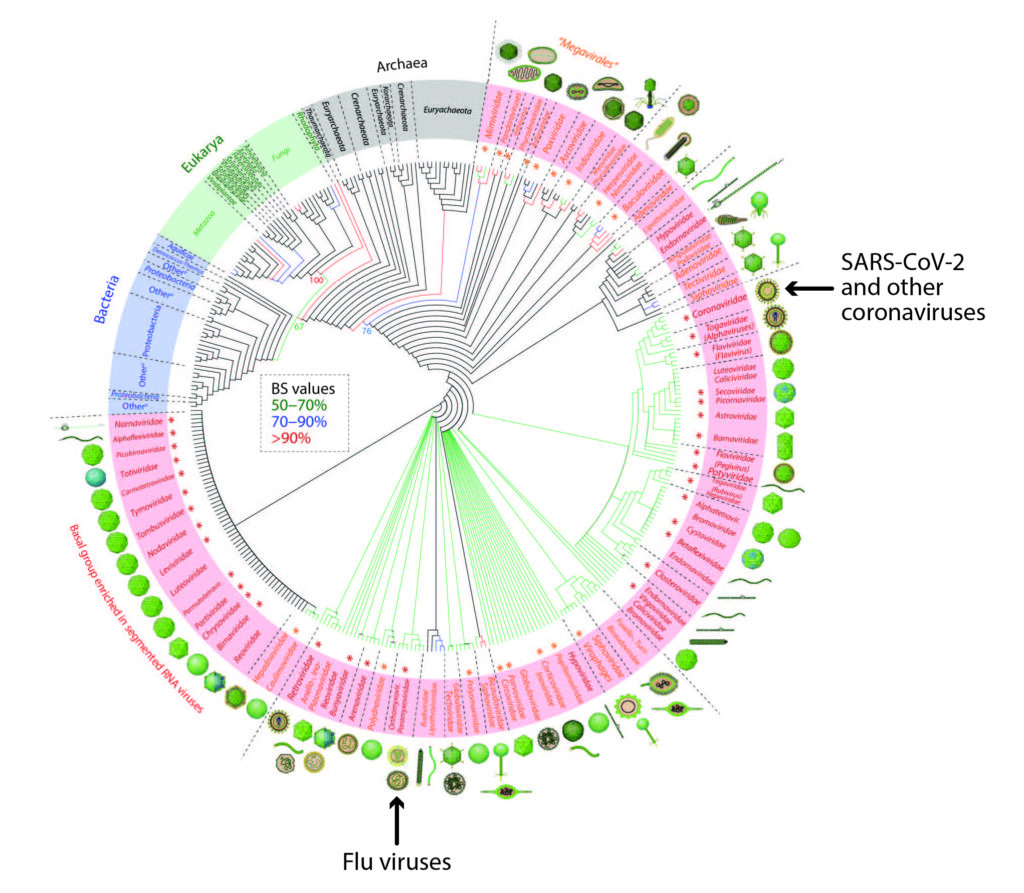
COVID is caused by the virus SARS-CoV-2, a member of the coronavirus clade. Viruses in this tight knit family infect humans and a range of other animals, causing respiratory infections, and in non-human animals, other illnesses like hepatitis and gastrointestinal disease. Bird flu, on the other hand, is caused by the influenza A virus, a member of the Orthomyxoviridae clade. All influenza viruses are a part of this clade, including the viruses responsible for regular seasonal flu in humans. While many different strains of influenza A circulate around the world, it is the rapidly spreading H5N1 strain, which normally infects birds, putting public health workers on high alert. As you can see from the evolutionary tree, or phylogeny, below, coronaviruses and flu viruses are not very closely related.

Despite their fairly distant relationship, H5N1 and SARS-CoV-2 do have an important similarity: both of their clades have genomes made of RNA, not DNA. DNA is a stable molecule, with two copies of all the information it encodes, one in each strand of the double helix. Single-stranded RNA, on the other hand, degrades more easily and has no back-up copy of its data. This means that RNA-based genomes are much more prone to mutations – mistakes in repair or copying – than are DNA-based genomes.
Correspondingly, RNA viruses have high mutation rates, which has an important implication for evolution. Mutations create genetic variation – genetic differences from one individual to the next – and it is among these genetic variants that natural selection sorts, with some variants leaving behind more descendants than others. Higher mutation rates mean more chances that random mutation will just happen to strike upon a variant that provides an advantage in a new situation – setting the stage for natural selection to produce adaptations quickly.
The potentially rapid pace of evolution in both coronaviruses and influenza viruses threatens public health. SARS-CoV-2 evolved adaptations that allow it to spread easily among humans and then quickly diversified into a Greek alphabet soup of strains – Alpha, Beta, Delta, etc. – which we now monitor for spread, transmissibility, virulence, and vaccine effectiveness. We track the evolution of influenza viruses in similar ways, a surveillance system that revealed the current outbreak of H5N1 as a risk. So far, H5N1 does not seem to be able to spread from person to person, only from non-human animals to humans. But new mutations could change that, potentially sparking a pandemic.
Unfortunately, influenza A has another genetic characteristic that further boosts its rate of evolution and the chances that it gains the ability to spread from person to person. While the genome of a coronavirus is one continuous strand of RNA, the genome of influenza A is encoded on eight separate segments of RNA. When the influenza virus enters a host’s cell, it releases all those bits of its genome to be copied by the host cell. The segment copies are then repackaged into many viral offspring. However, when two different viruses invade the same host cell, their viral segments can get mixed up, with offspring having a combination of segments from the two different “parent” viruses. This process is called reassortment, and it provides yet another source of genetic variation that natural selection can act upon. Through reassortment, even very different influenza strains can easily exchange big chunks of genetic information. So, for example, if a farmworker were infected by both a regular seasonal flu virus (which has genetic information that allows it to be transmitted from one person to another) and bird flu, the two strains could easily swap some gene versions, resulting in potentially dangerous hybrid viruses. Such reassorted viruses have been responsible for prior influenza pandemics and millions of deaths worldwide.
Another evolutionary similarity between H5N1 influenza virus and SARS-CoV-2 relates to their ancestral hosts. Each is what’s known as a zoonotic disease – a pathogen that evolves to infect humans after previously infecting only non-human animals. While the origin of SARS-CoV-2 is not completely certain, we do know that close relatives of this viral strain normally circulate among bats. H5N1 naturally circulates among birds. In both cases, non-human animals host a vast number of diverse viral strains, and close contact between humans and those animals presents a risk, not just of the virus infecting a person, but of a virus evolving the ability to spread from one human to another, breaking through a critical barrier on the path to a pandemic. For SARS-CoV-2, this process may have occurred in an urban market where live wild animals were sold, mixing with each other and with all the people attending and working at the market. For H5N1, the worry is that conditions on a dairy or poultry farm could be setting the stage for the virus to make this same evolutionary leap.
While SARS-CoV-2 and H5N1 are similar in many ways, their evolutionary destinies need not be. Reducing contact between humans and infected animals reduces the chances that we spark the pandemic potential of H5N1 in the first place. For example, in the U.S., new guidance from the Centers for Disease Control and Prevention aims to decrease animal workers’ exposure to potentially infected animals. Further, the lessons of the COVID-19 pandemic could help us snuff out a new flu virus before it can no longer be contained. While bird flu has the potential to kill people, it’s not yet clear how deadly a bird flu pandemic would be. And of course, the speed with which we can develop new vaccines dramatically improved over the course of the coronavirus pandemic. Another devastating pandemic is certainly possible, but it is not inevitable if we take action and build on the lessons of the past.
Primary literature:
- Lycett, S.J., Duchatel, F., and Digard, P. (2019). A brief history of bird flu. Philosophical Transactions of the Royal Society London B Biological Sciences. 374:20180257 Read it »
News articles:
- A summary of the current risks of H5N1 from the New York Times
- An interview on the recent evolution of H5N1 from Penn State
Understanding Evolution resources:
- Describe a possible evolutionary change in the H5N1 virus that scientists and public health workers are concerned could lead to a pandemic.
- In your own words, explain what a mutation is.
- According to the article above, what two differences between RNA and DNA are responsible for their different mutation rates?
- Review some background information on natural selection. Identify which of the four steps on that page helps explain the quicker pace of evolutionary change in RNA-based viruses in comparison to DNA-based viruses. Explain your reasoning.
- Describe the additional source of genetic variation, beyond direct mutations to their RNA genomes, that influenza A viruses have in comparison to coronaviruses.
- Describe one measure addressed in the article above that could help prevent a virus that infects non-human animals from evolving the ability to be passed among human hosts.
- Teach the basics of natural selection: In this classroom activity for grades 9-12, students learn about variation, reproductive isolation, natural selection, and adaptation through this version of the bird beak activity.
- Teach about the causes of mutation: In this article (and the linked assignments and student readings) for the college level, students examine and interpret data that scientists use to study the causes of mutations that matter most for evolution. Use the tabs at the bottom of the feature to find related videos, assignments, and lessons to build this example into a lesson sequence on mutation.
- Teach about avian flu: This news story for high school and college students addresses previous outbreaks of avian flu.
- Bohannon, M. (2025). Here’s why egg prices are so high – as Waffle House implements egg surcharge. Forbes. Retrieved February 4, 2025, from Forbes (https://www.forbes.com/sites/mollybohannon/2025/02/04/heres-why-egg-prices-are-so-high-as-waffle-house-implements-egg-surcharge/)
- Centers for Disease Control and Prevention. (January 6, 2025). First H5 bird flu death reported in United States. CDC Newsroom. Retrieved February 4, 2025, from the CDC (https://www.cdc.gov/media/releases/2025/m0106-h5-birdflu-death.html).
- Howard, L. (April 25, 2024). 1 in 5 milk samples from grocery stores test positive for bird flu. Why the FDA says it’s still safe to drink. UC Davis Health. Retrieved February 4, 2025, from UC Davis (https://health.ucdavis.edu/news/headlines/grocery-store-milk-tests-positive-for-bird-flu-why-the-fda-says-its-still-safe-to-drink/2024/04).
- Lycett, S.J., Duchatel, F., and Digard, P. (2019). A brief history of bird flu. Philosophical Transactions of the Royal Society London B Biological Sciences. 374: 20180257.
- Sanjuán, R., Nebot, M. R., Chirico, N., Mansky, L. M., and Belshaw, R. (2010). Viral mutation rates. Journal of Virology. 84: 9733-48.
- Temmam, S., Vongphayloth, K., Baquero, E., … and Eloit, M. (2022). Bat coronaviruses related to SARS-CoV-2 and infectious for human cells. 604: 330–336.
