
Many fossils speak for themselves: it’s hard to mistake a T. rex femur for a strangely shaped boulder, or an elaborate fossil of Archaeopteryx for random cracks in limestone. But other cases are not so clear cut. For example, examine the photo at right. Is this an imprint of some prehistoric fern? It might look that way, but this structure (called a dendrite) is not a fossil and is produced by the crystallization of one mineral on another, forming a branching pattern.
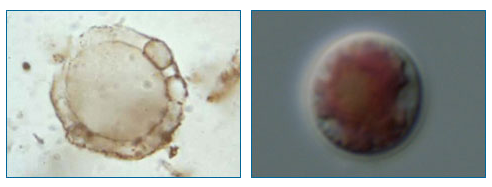
The problem of determining what is and is not a fossil can be especially difficult when it comes to ancient microfossils. Because these fossils are of relatively simple organisms, such as bacteria and single-celled algae, without much in the way of identifying features (like leaves or horns), it can be a challenge to demystify them — to figure out what sort of living thing they represent, if indeed, they represent any living thing at all. For example, the microscopic fossil shown on the left below comes from 2 billion year old rock. It is only 20 microns long — that’s less than the width of a human hair! This fossil looks similar to a modern unicellular red algae, Porphyridium (shown on the right below), but from appearances alone, it can be hard to tell what organism the fossil really represents.
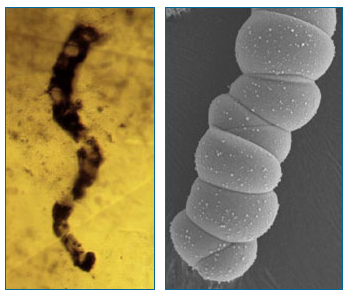
Compounding the problem, modern microorganisms can sometimes invade minute pores in ancient rocks, making the identification of real fossils tricky. Even worse, geologic chemical reactions can sometimes produce tiny structures resembling simple bacteria and algae — such as the one shown below, which was cooked up by geologists in a lab. If these same reactions occurred on ancient Earth, they might have left behind traces that would easily be mistaken for fossils. With all these red herrings around, how can a paleontologist figure out what is and is not a fossil?
Luckily, modern technology and scientific knowledge have come to the rescue:
- Improved microscopic and imaging techniques sometimes allow scientists to zoom in on these fossils to identify hallmarks of life, such as the cell wall.
- Advanced chemical analysis tools can compare the chemical makeup of the fossil itself to the surrounding rock to note any indication that the structure was once alive. These techniques for example, can help identify very tiny samples of kerogen, the organic material into which living things decay.
- Elements come in forms with different weights, called isotopes. Carbon-12 and carbon-13 (the heavier of the two) are both common on Earth, but living things prefer to use carbon-12. Sensitive techniques can determine whether a rock or putative fossil contains more carbon-12 than expected, suggesting that the material may once have been alive.
In order to figure out if a microfossil is really a fossil, paleontologists use the tools above, along with other observations, to evaluate the following criteria:
- Does the alleged fossil look “life-like?” In other words, does it have morphological structures consistent with living things?
- Based on geologic information, did the “fossil” form in an environment that could have sustained life and then preserved a fossil?
- Does the “fossil” have a biogeochemical make-up that suggests it was once alive? For example, is it high in carbon-12?
Putative microfossils that meet all of these criteria are good candidates for the real thing!
