Paleontologists and geologists try to answer all sorts of questions about mass extinctions:
- Which species went extinct and which survived?
- What geographic areas and ecosystems were most affected?
- When and over what period of time did the mass extinction occur?
These questions may seem simple enough, but they can be tricky to answer.
Establishing snapshots of life before and after a mass extinction is challenging for many reasons. We have access to only a small subset of all the fossils that might be preserved in fossil record. And for the fossils we do have, it is often difficult to identify a species and genus, let alone figure out whether it had any descendents that survived to later time periods. Adding a further challenge, whole groups of organisms do not have body parts that fossilize well and so are largely absent from the fossil record.
Radioisotopic dating in a nutshell
To date a rock, geologists study minerals in the rock that contain radioactive elements and that were formed very close to the time that the rock was deposited. These elements decay away at known rates and so can be used as a sort of clock to determine the age of the rocks. Geologists measure how much of these radioactive elements have decayed away and, from that, work backwards to figure out how old the rock must be. It works like this:
- As certain minerals form, radioactive elements (like uranium and potassium) may be bound up in the structure of the mineral. Around the time of their formation, these minerals became part of a rock (for example, in volcanic rocks).Over time, the radioactive elements decay. For example, over many millions of years, uranium decays into lead and potassium decays into argon. Scientists have determined the rate at which this happens by studying these elements experimentally.
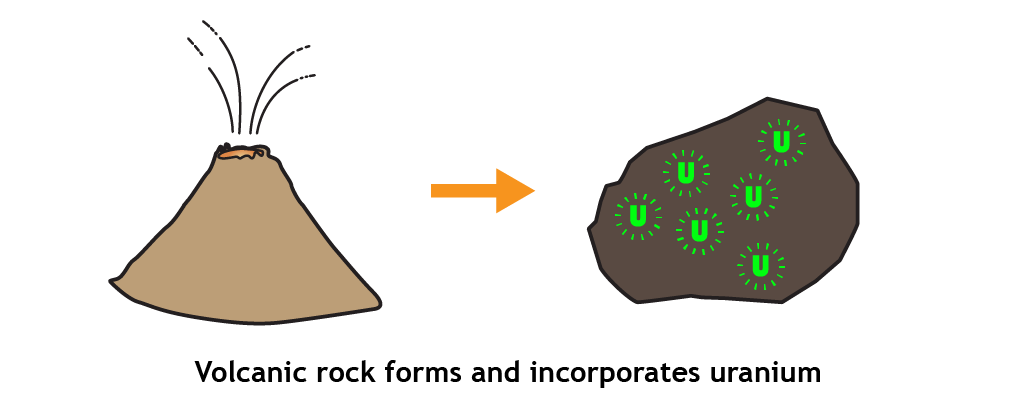
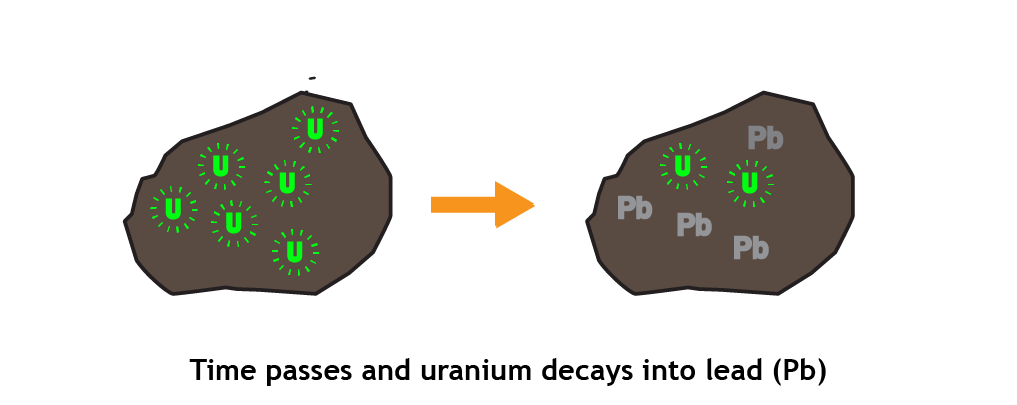
- Over time, the radioactive elements decay. For example, over many millions of years, uranium decays into lead and potassium decays into argon. Scientists have determined the rate at which this happens by studying these elements experimentally.
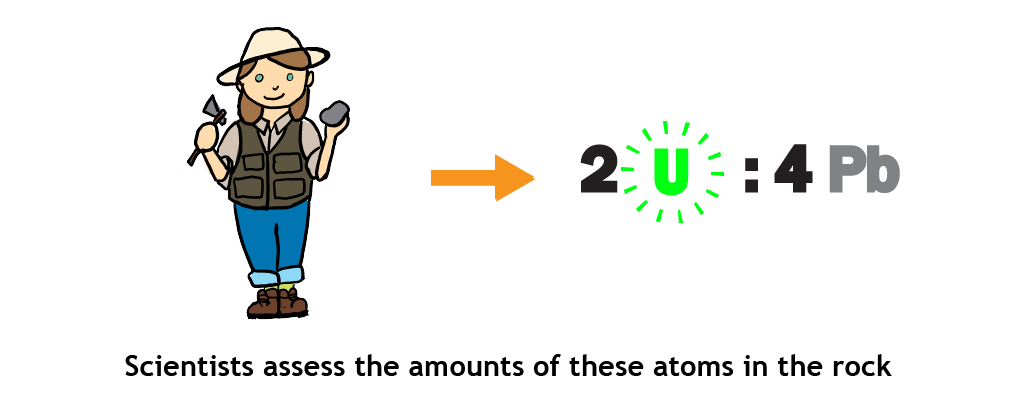
- Scientists collect samples of the rock, extract the minerals containing the radioactive elements, and determine the ratio of the radioactive element to its decay product present in the mineral.
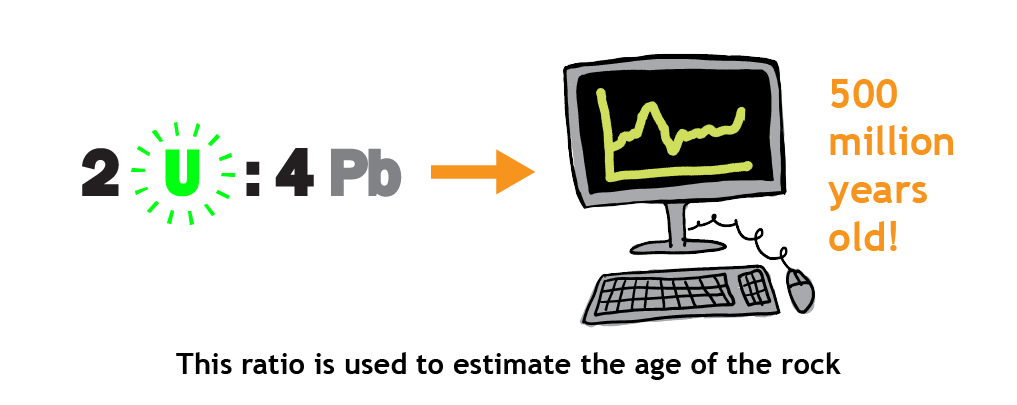
- Based on this ratio and the known rate of decay for that element, scientists calculate the age of the rock — i.e., how much time must have passed for the mineral to contain the observed ratio of radioactive element and product. This is usually expressed as an age range — for example, 252.4 million years ago plus or minus 0.3 million years. This means that scientists can be very certain that the rock dates to sometime between 252.1 million years ago and 252.7 million years ago.
Radiocarbon dating works using the same basic principles, but is used to date organic material and relies on a different element (carbon), whose radioactive form (known as carbon-14) decays much more quickly. This speedy decay rate means that carbon can only be used to estimate ages up to around 60,000 years.
Still have questions? Get The nitty gritty on radioisotopic dating.
