Evolutionary biologists can help uncover clues to new ways to treat or vaccinate against HIV. These clues emerge from the evolutionary origins of the virus, how human populations have evolved under pressure from other deadly pathogens, and how the virus evolves resistance to the drugs we’ve designed. Controlling the disease may be a matter of controlling the evolution of this constantly adapting virus.
 The human immunodeficiency virus (HIV, shown here budding from a white blood cell) is one of the fastest evolving entities known. It reproduces sloppily, accumulating lots of mutations when it copies its genetic material. It also reproduces at a lightning-fast rate — a single virus can spawn billions of copies in just one day. To fight HIV, we must understand its evolution within the human body and then ultimately find a way to control its evolution.
The human immunodeficiency virus (HIV, shown here budding from a white blood cell) is one of the fastest evolving entities known. It reproduces sloppily, accumulating lots of mutations when it copies its genetic material. It also reproduces at a lightning-fast rate — a single virus can spawn billions of copies in just one day. To fight HIV, we must understand its evolution within the human body and then ultimately find a way to control its evolution.
Taking an evolutionary perspective on HIV has led scientists to look in three new directions in their search for treatments and vaccines:
 1. What are the evolutionary origins of HIV?
1. What are the evolutionary origins of HIV?
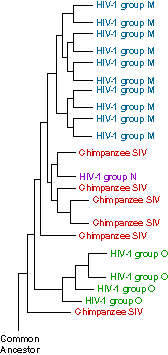
HIV, like any evolving entity, has been deeply marked by its history. Scientists studying the evolutionary history of HIV found that it is closely related to other viruses. Those viruses include SIVs (simian immunodeficiency viruses), which infect primates, and the more distantly related FIVs (the feline strains), which infect cats.
However, studies of these related viral lineages showed something surprising: primates with SIV and wild cats with FIV don’t seem to be harmed by the viruses they carry. If scientists can figure out how non-human primates and wild cats are able to live with these viruses, they may learn how to better treat HIV infections or prevent them altogether.
The diagram shows some of the evolutionary history of HIV as we know it today. An ancestral virus (bottom) evolved into strains that infected chimpanzees (SIV). Over time, new strains began to infect humans (HIV).
2. Why are some people resistant to HIV?
HIV is by no means the first plague that human populations have weathered. Many pathogens have deeply affected our evolutionary history. In fact, the human genome is littered with the remnants of our past battles with pathogens — and one of these remnants, a mutation to a gene called CCR5, may lead researchers to a new treatment for HIV.
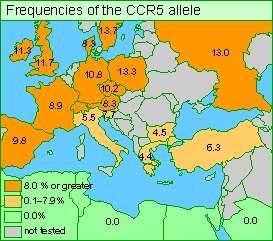 The mutant CCR5 allele probably began to spread in northern Europe during the past 700 years when the population was ravaged by a plague. (It may have been bubonic plague or some other pathogen; research on this topic continues.) The mutant CCR5 probably made its bearers resistant to the disease, and so its frequency increased.
The mutant CCR5 allele probably began to spread in northern Europe during the past 700 years when the population was ravaged by a plague. (It may have been bubonic plague or some other pathogen; research on this topic continues.) The mutant CCR5 probably made its bearers resistant to the disease, and so its frequency increased.
In some parts of Europe today, up to 20% of the population carry at least one copy of the protective allele. However, the populations of Asia and Africa were not exposed to the same epidemics; very few Asians and Africans now carry the allele (see map above). Thus, CCR5 is fairly common in northern Europe but its frequency diminishes as one moves south, and the mutation is rare in the rest of the world.
We now know that the mutant CCR5 allele has an unexpected side effect: it confers resistance to HIV. Scientists hope that studying this by-product of past selection will help them develop new treatments for the HIV epidemic ravaging human populations today.
3. How can we control HIV’s evolution of resistance to our drugs?
HIV evolves so quickly that it evolves right out from under our treatments. When a patient begins taking an HIV drug, the drug keeps many of the viruses from reproducing, but some survive because they happen to have a certain level of resistance. Because of HIV’s speedy evolution, it responds to selection pressures quickly: viruses that happen to survive the drug are favored, and resistant virus strains evolve within the patient, sometimes in just a few weeks. However, basic evolutionary theory points out a way that this evolution of resistant viral strains can be delayed. Patients are prescribed “drug cocktails” — several different HIV drugs taken together.
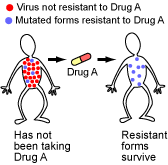 When taking any single drug, it is fairly likely that some mutant virus in the patient might happen to be resistant, survive the onslaught, and spawn a resistant lineage.
When taking any single drug, it is fairly likely that some mutant virus in the patient might happen to be resistant, survive the onslaught, and spawn a resistant lineage.
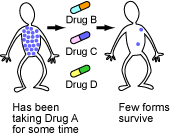 But the probability that the patient hosts a mutant virus that happens to be resistant to several different drugs at the same time is much lower. Although multiple-drug-resistant HIV strains do eventually evolve, drug cocktails delay their evolution.
But the probability that the patient hosts a mutant virus that happens to be resistant to several different drugs at the same time is much lower. Although multiple-drug-resistant HIV strains do eventually evolve, drug cocktails delay their evolution.
An evolutionary trade-off
If a patient is already infected with a drug-resistant HIV strain, basic evolutionary theory has also pointed out a way to make the drug useful again. Studies of the evolution of resistance often show that you don’t get something for nothing. Specifically, it “costs” a pest or pathogen to be resistant to a pesticide or drug. If you place resistant and non-resistant organisms in head-to-head competition in the absence of the pesticide or drug, the non-resistant organisms generally win.
Consider a patient who takes a particular drug and winds up with viruses resistant to the drug. If the patient stops taking the drug for a while, evolutionary theory predicts that her viral load will evolve back towards a non-resistant strain. If she then takes very strong doses of the drug, it may be able to halt the replication of those non-resistant viruses and reduce her viral load to very low levels.
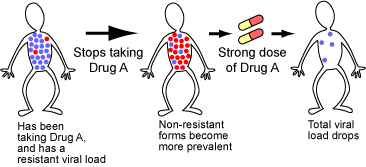
This therapy has shown early, promising results — it may not eliminate HIV, but it could keep patients’ virus loads low for a long time, slowing progression of the disease.
Ultimately, understanding the evolutionary history of HIV and its pattern of evolutionary change may help us control this disease.
